Case report
A female infant was born small for gestational age (2.46 kg) at 36 weeks to a father of Sudanese descent and a Caucasian mother. She had feeding difficulties, vomiting, diarrhea, and failure to thrive during the first four weeks of life. She suffered repeated episodes of sepsis with Staphylococcus aureus, Escherichia coli, and Serratia marcescens. At the early age of 3 months she was found to have lymphopenia. At the age of 5 months she was transferred to another hospital and again had repeated episodes of sepsis with Serratia marcescens, streptococcal pneumoniae, and Staphylococcus aureus. She was then transferred to our institution for further evaluation of her immunity. The patient developed pneumonitis due to para influenza virus and her respiratory status deteriorated gradually. In spite of maximal support treatment in the pediatric intensive care unit she died of hypoxic cardiac arrest at the age of 1 year.
Microarray analysis, karyotype, and chromosome breakage studies were normal. Genetic testing showed no abnormalities in RMRP, CD25, CD3ɛ, CD3δ, STAT1, IL-7Rα, IL-10, IL-10Rα, and IL-10Rβ.
Immune evaluation
Immune evaluation revealed a low lymphocyte count with lymphocyte immunophenotyping showing CD3
+ 791 cells/µL, CD4
+ 365 cell/µL, CD8
+ 265 cell/µL, and CD56
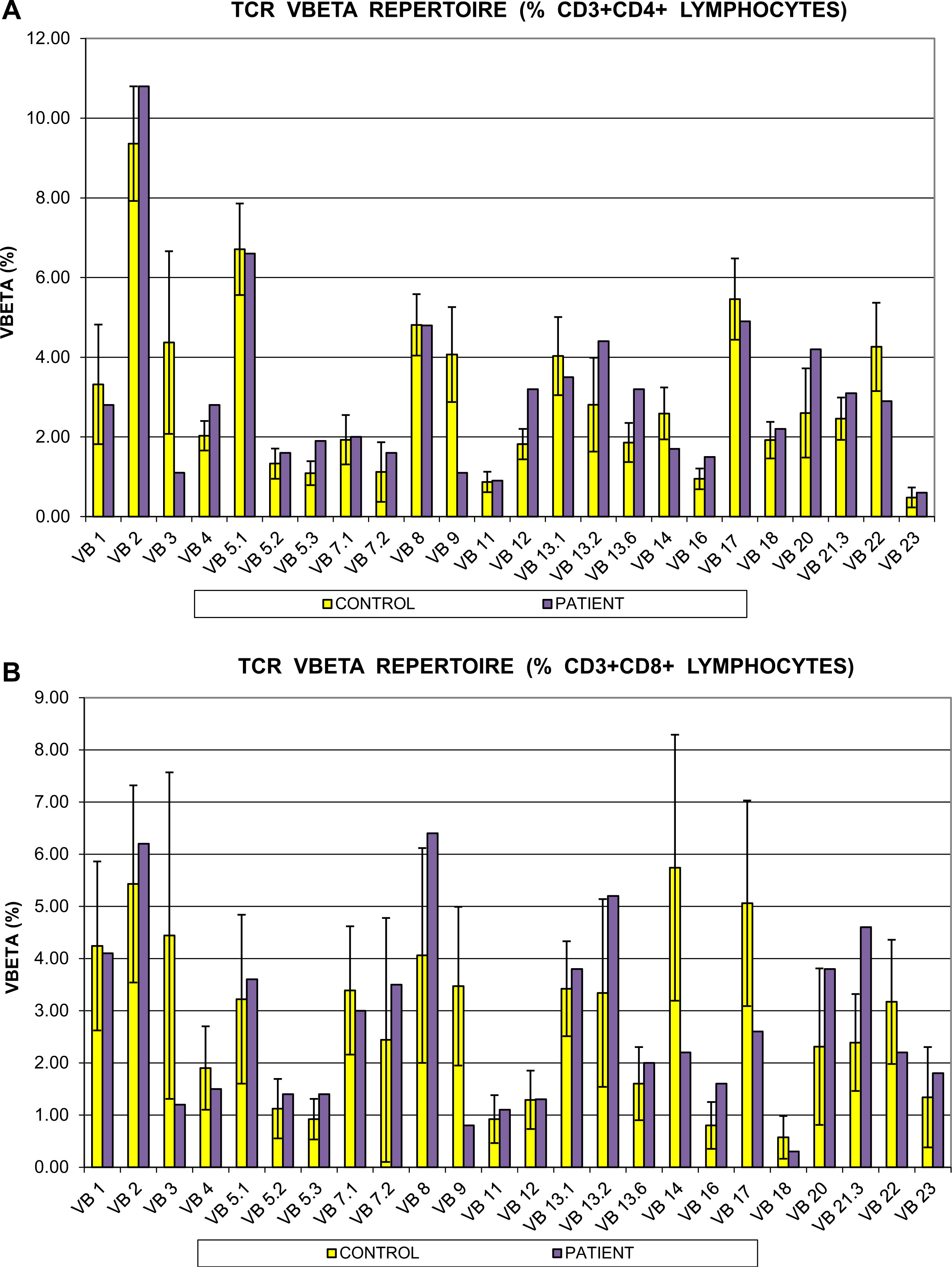
+ 17 cell/µL cells. In vitro responses to PHA and anti-CD3 antibody stimulation were markedly reduced at <40% and <10% of controls, respectively. T-cell repertoire was also abnormal. The CD4
+ cell compartment showed reduced representation of the Vβ3, Vβ9, and Vβ14 families, whereas Vβ5,3, Vβ13,2, and Vβ20 were overrepresented. Repertoire skewing was more prominent in CD8
+ cells, whereby Vβ3, Vβ9, Vβ14, Vβ17, and Vβ23 families were underrepresented, with overrepresentation of Vβ21.3 only (
Figure 1).
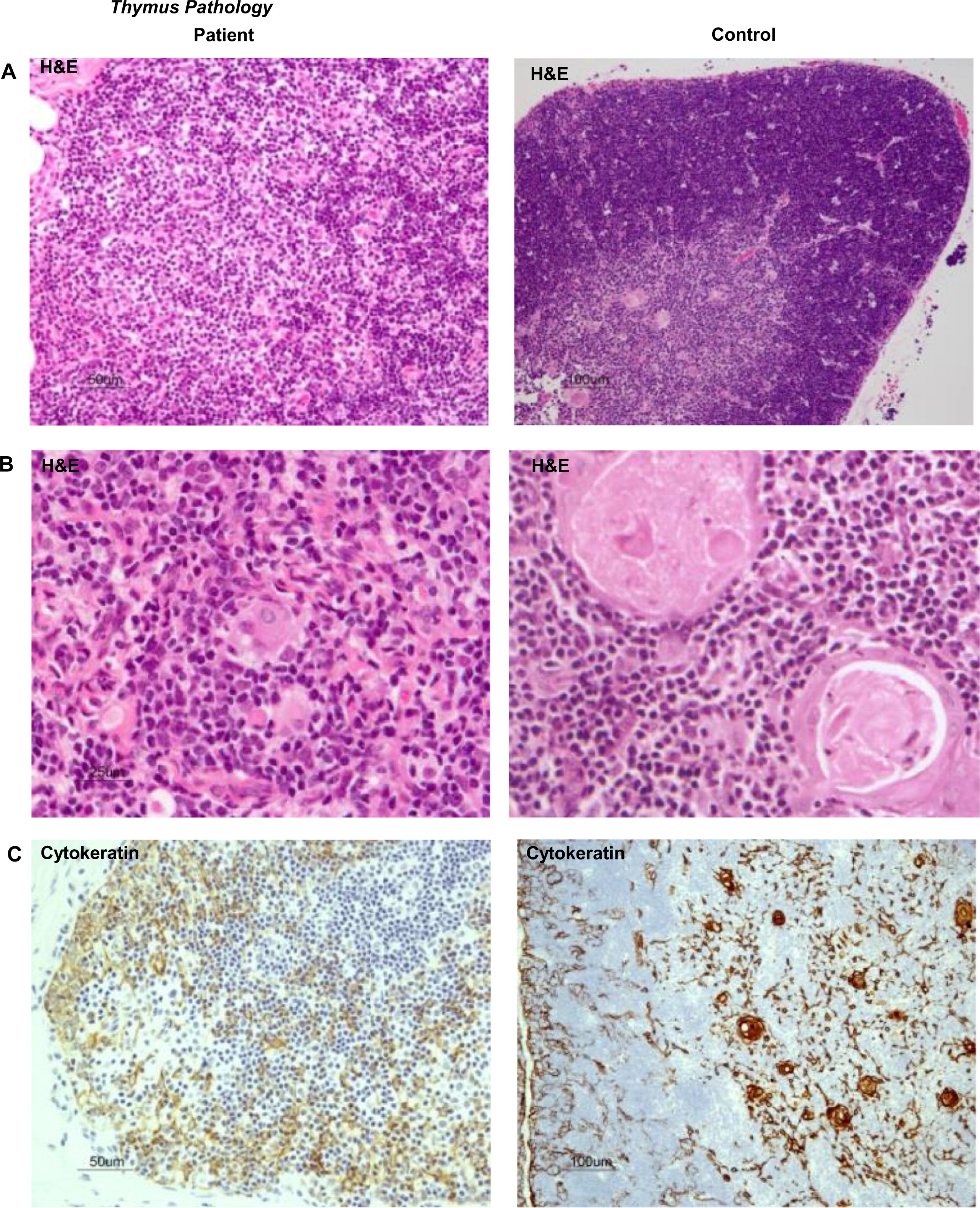
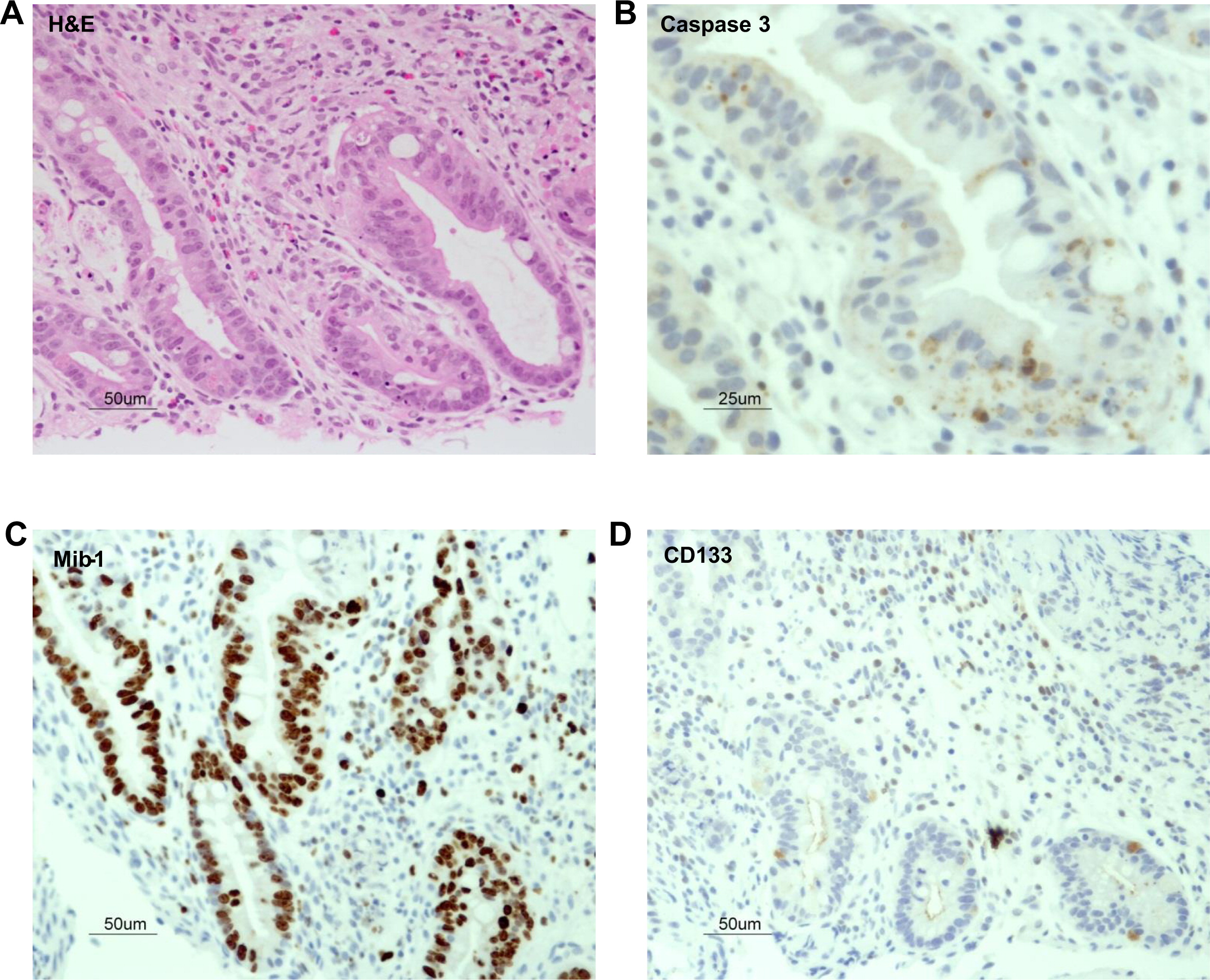
These results predicted impaired function of the thymus. We performed a thymus biopsy that showed a small and depleted gland. Most Hassall's corpuscles were small and poorly formed and their total number was markedly diminished when compared with a control (11 vs. 57 per field), thus clearly suggesting a primary immunodeficiency (
Figures 2A and 2B). Corticomedullary demarcation was also compromised, with a reduced number of thymocytes in the cortical regions. Cytokeratin staining confirmed the rarity of Hassall's corpuscles and their immature nature (
Figure 2C). Immunohistochemistry for CD3, CD4, and CD8 appear to show positive staining, but overall numbers were reduced in full agreement with H&E staining (
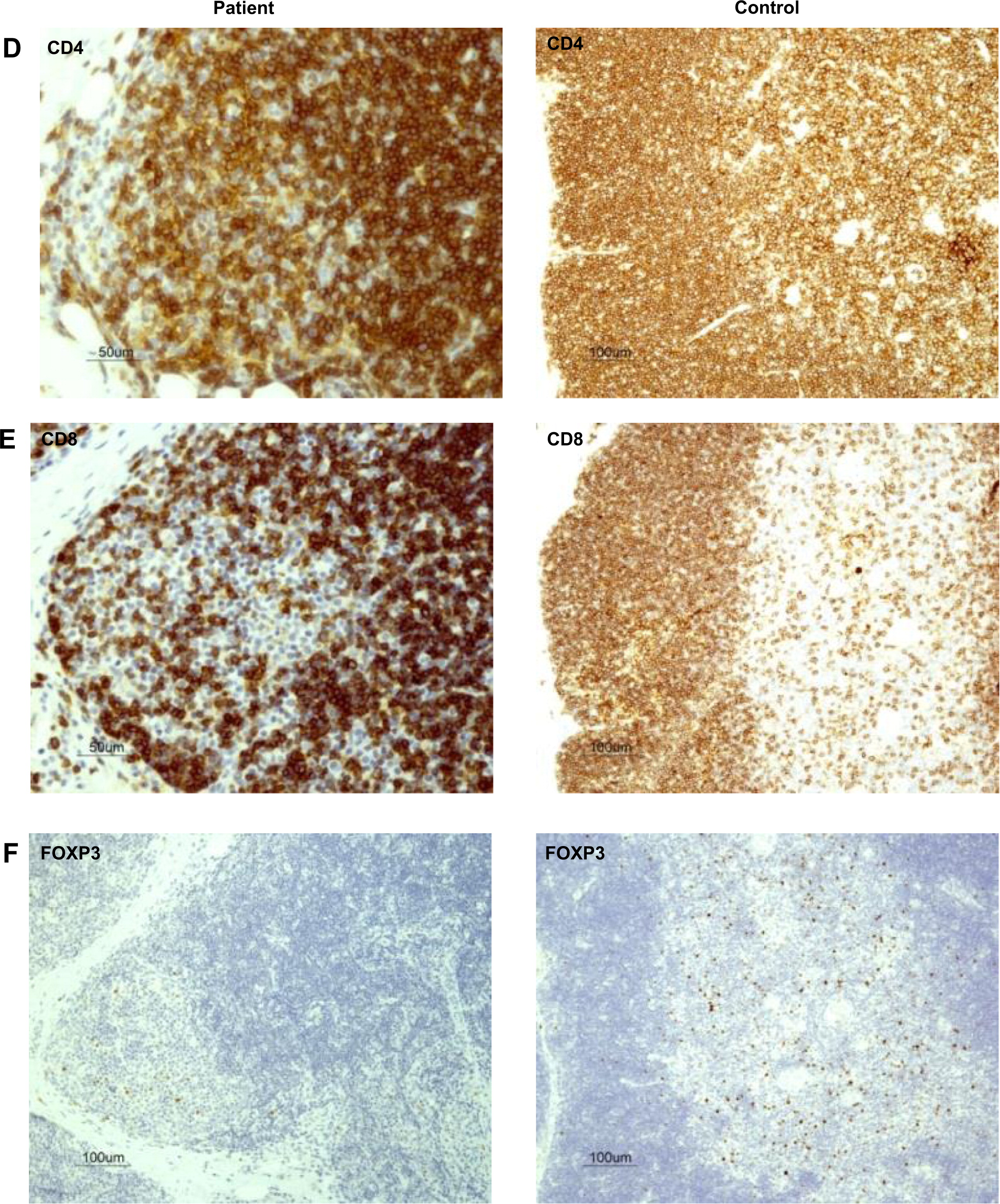
Figures 2D and 2E). Remarkably, the number of FOXP3
+ regulatory cells was also drastically reduced in the patient's thymus (
Figure 2F). In support of the notion of a dysfunctional thymus, T-cell receptor excision circle (TREC) levels, which represent new thymus emigrants, were reduced (275 ± 120 in three different determinants).
Immunoglobulins were all low (IgG < 0.6 g/L, IgA <0.2 g/L, and IgM < 0.2 g/L) and specific antibody production in response to vaccination was undetectable.
Lung pathology
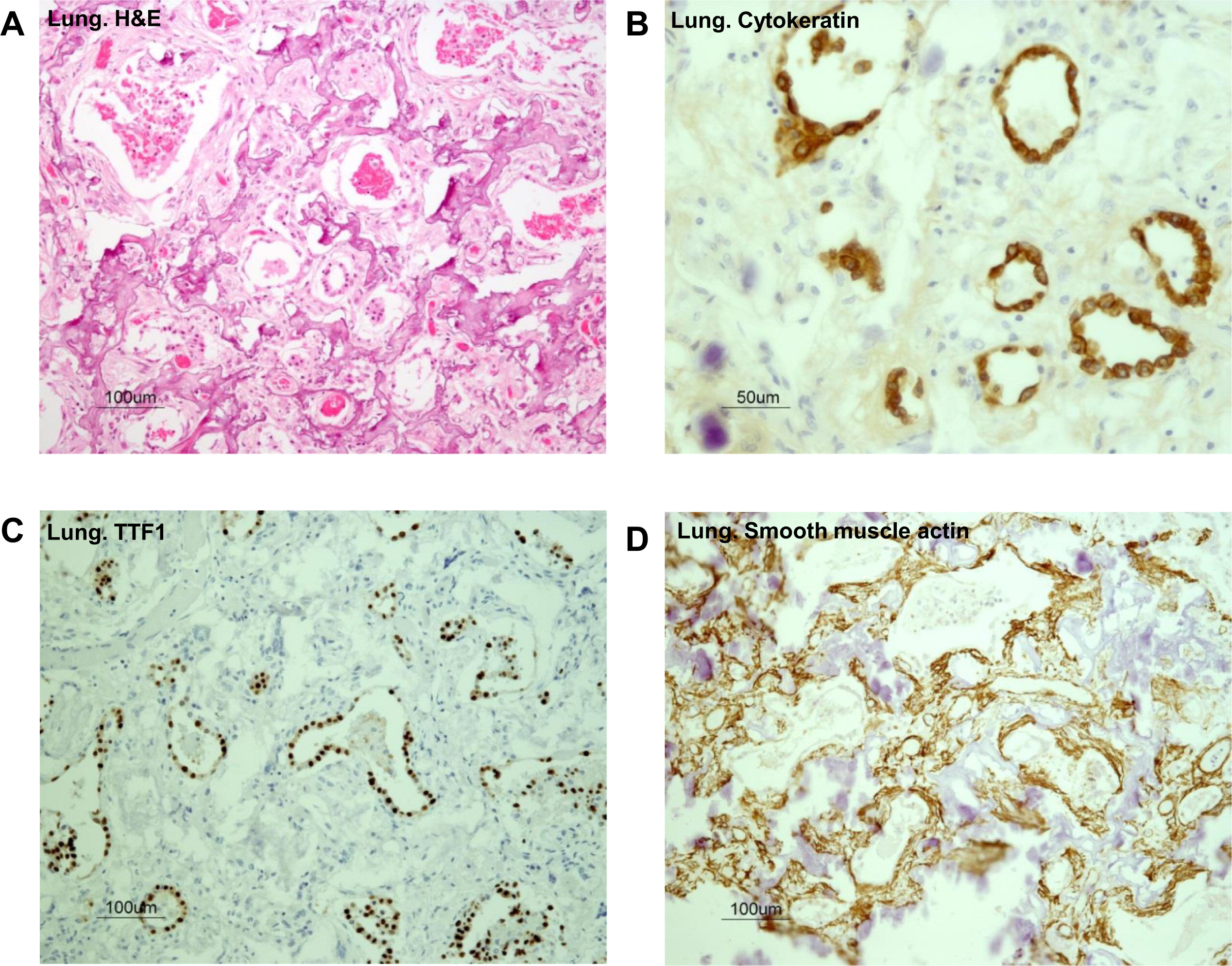
Evaluation of the autopsy lung pathology revealed diffuse alveolar damage and severe interstitial pulmonary fibrosis with honeycomb transformation of the pulmonary parenchyma (
Figure 3A). There was marked reduction of alveoli and all were structurally remodeled into small round air filled microcysts that were lined with low cuboidal type-2 pneumocytes. These cells were found positive for pan cytokeratin and TTF-1 by immunostaining (
Figures 3B and 3C). In addition, diffuse micro-dendriform pulmonary ossification was present. Dendriform networks of thin spicules of metaplastic osseous tissues were formed within the abnormal thick and fibrotic interalveolar septae (
Figure 3A). The metaplastic osseous tissues arose within the stroma that contained smooth muscle actin expressing myofibroblasts (
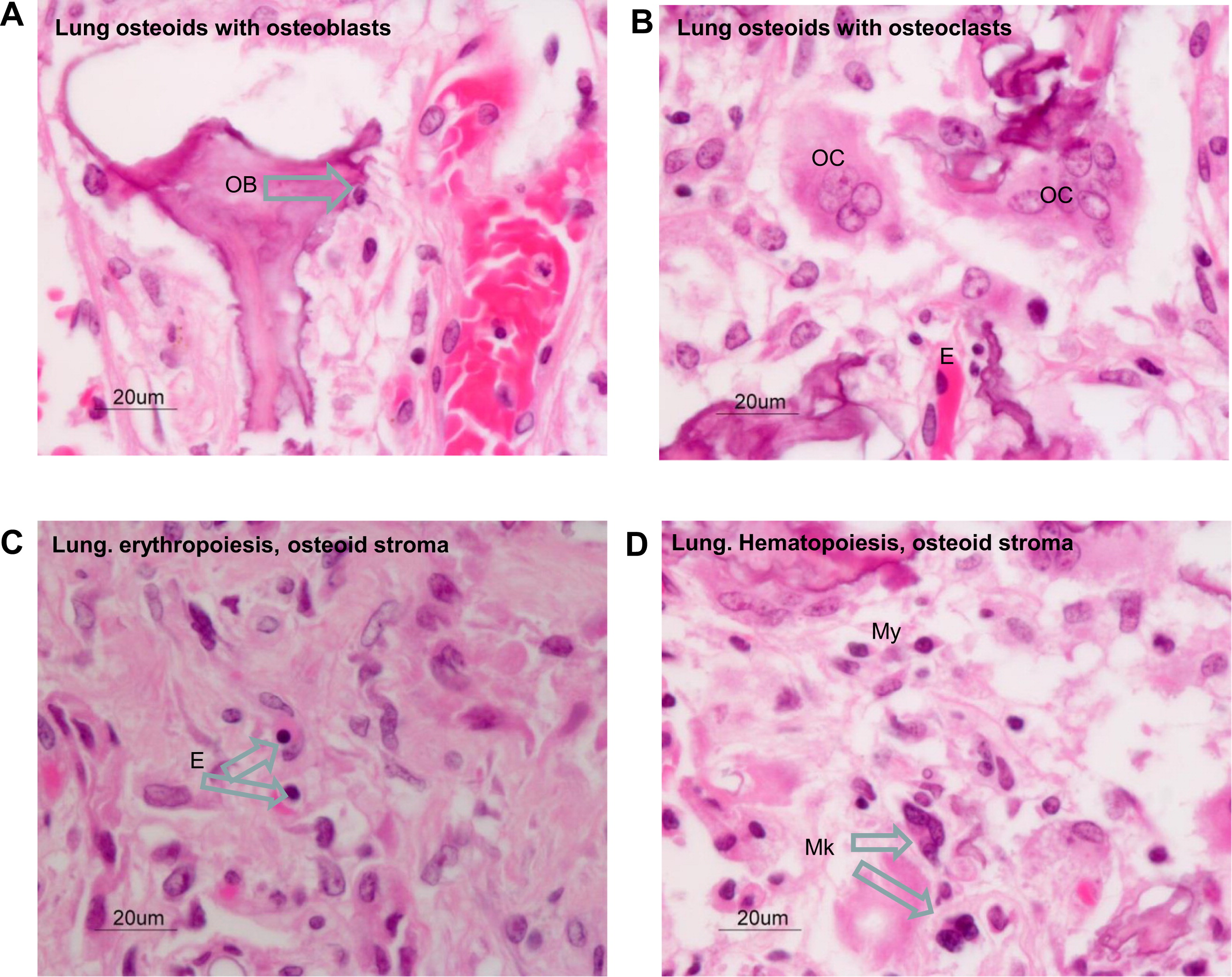
Figure 3D). Osteoblasts were associated with the bone spicules, and many of them showed active remodeling by osteoclasts (
Figures 4A and 4B). It appeared that this osteogenic activity was in its initial stages of bone marrow development. Within the fibrotic stroma there were clear foci of hematopoeisis, such as microscopic aggregates of para-trabecular progenitor myeloid, erythroid, and megakaryocytic cells (
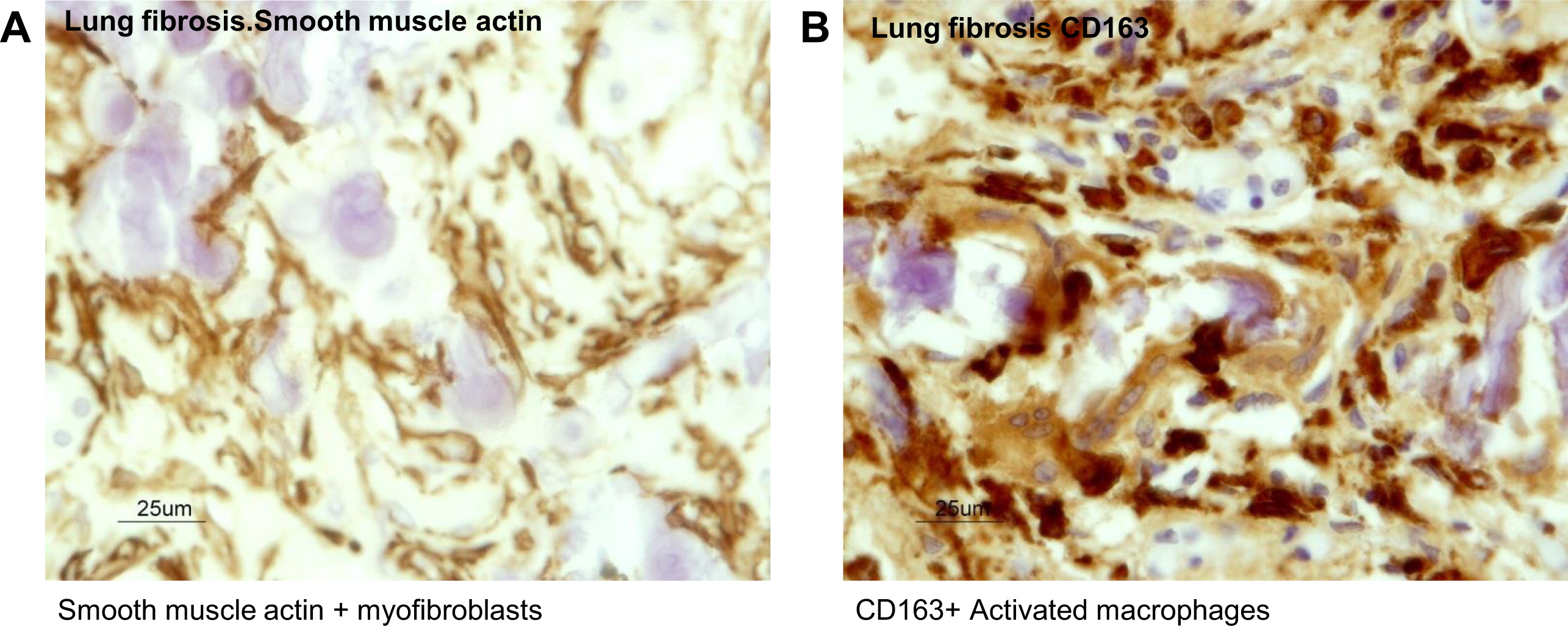
Figures 4C and 4D). Analyses of the cell types within the microenvironment of the fibrotic interalveolar pulmonary interstium, which showed osteogenesis, demonstrated a close association between smooth muscle actin-positive myofibroblasts and macrophages that expressed the activation antigen CD163 (
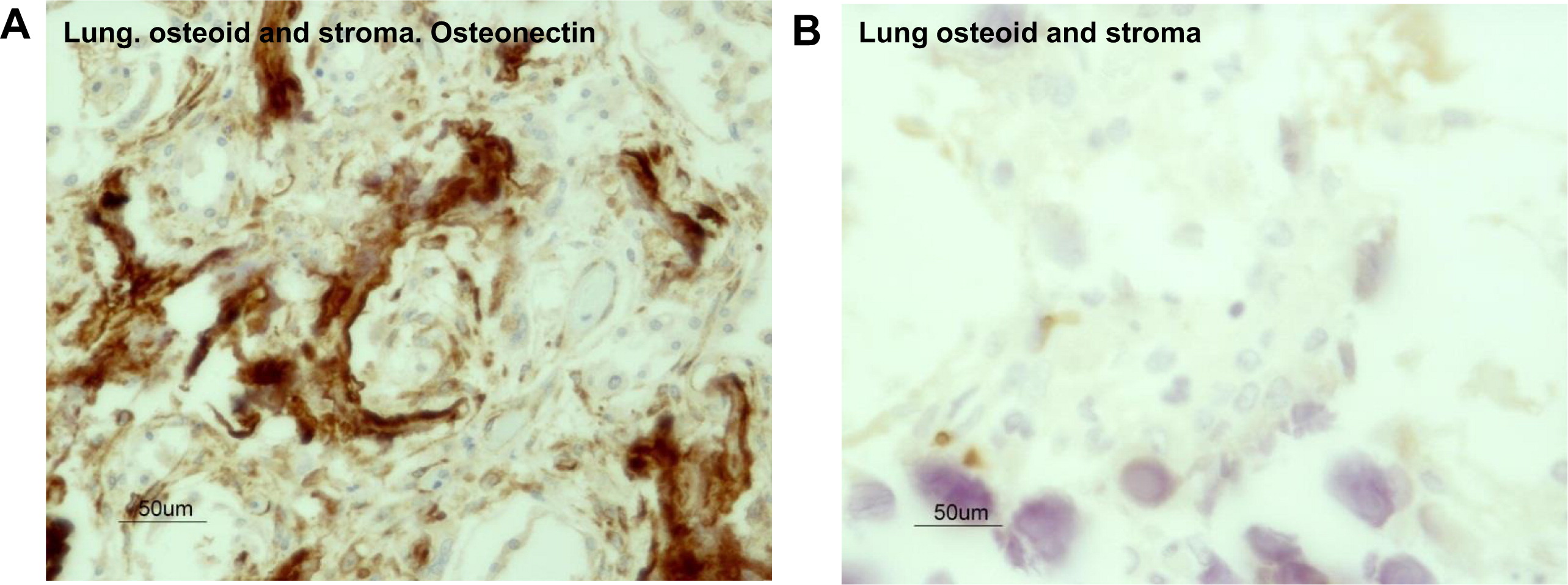
Figures 5A and 5B respectively). Immunostaining for the presence of osteonectin, one of the major noncollagenous proteins of bone associated with osteogenic differentiation from myofibroblasts, showed that it was expressed in the dendriform bone spicules (
Figure 6A) as well as in the myofibroblasts in the vicinity of the bone spicules. Immunostaining for T cells with CD3 showed that this microenvironment was devoid of T cells (
Figure 6B).
There were no histological features of bronchiolitis obliterans and no residual infiltrates of chronic inflammatory cells were seen within the fibrotic pulmonary interstitium. No viral cytopathic changes were found and stains for fungi and bacteria and immunostains for adenovirus, cytomegalovirus, and respiratory syncytial virus were all negative. DNA amplification by polymerase chain reaction detected parainfluenza A (consistent with patient's history).
Genetic analysis
To identify the underlining genetic cause of this disorder we employed whole genome sequencing. The whole genome of the patient was sequenced using the Complete Genomics Inc. (Mountain View, CA) platform.
The resulting sequencing run had >97.5% of the human genome covered by unique reads with depth 5× or greater and >95.7% with depth 10× or greater. The coding sequence coverage displayed only slightly higher percentages.
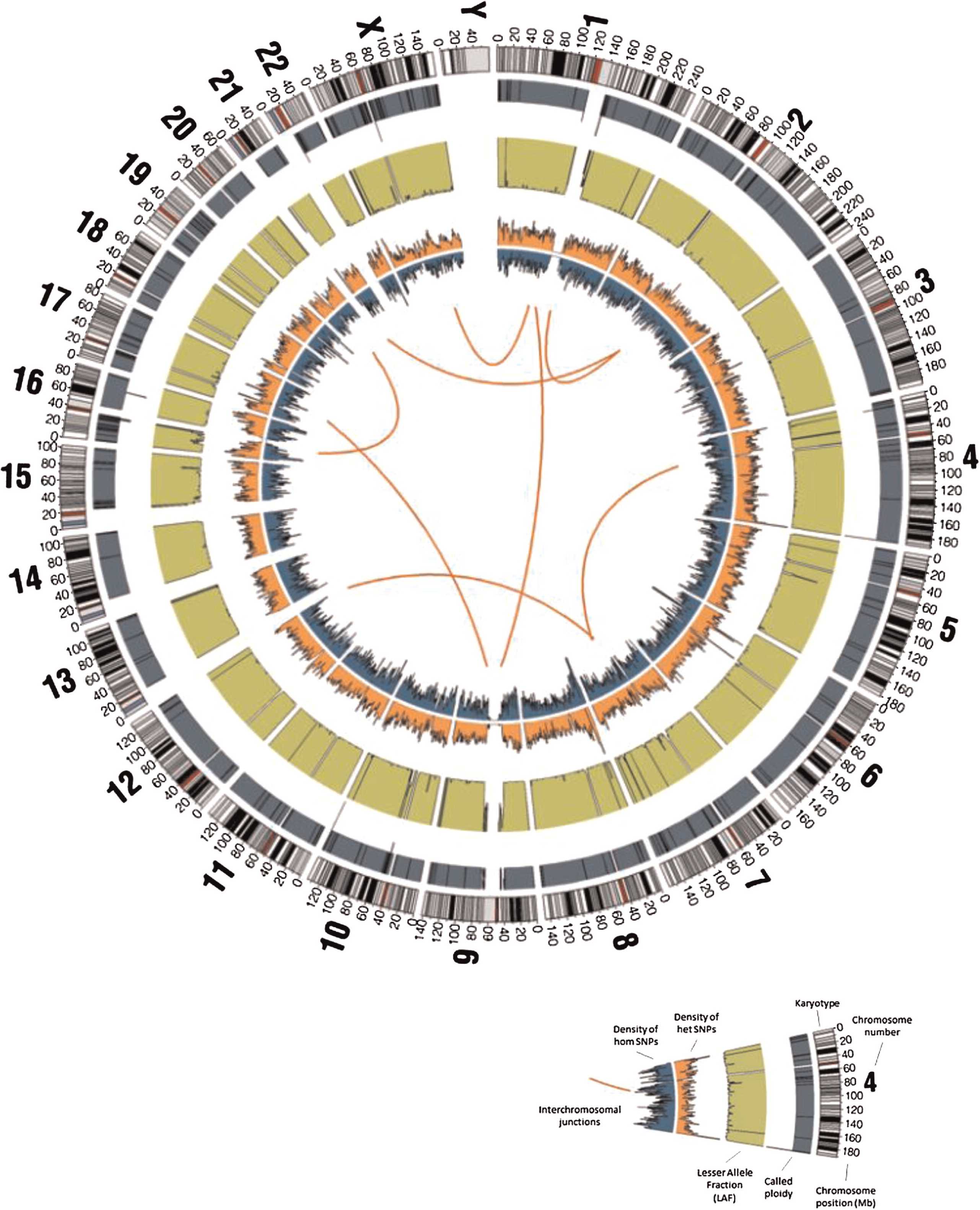
Of the 4 656 733 variants that were called (including “half calls” where only one allele is resolved), 4 161 694 (89.4%) passed CGI's default quality filters and 3 776 872 (81.1%) passed the more stringent filters. Of the variants passing quality filters, 24 512 and 22 389 (about 0.6% in both cases) were coding or splicing, respectively; of these, 2758 (11.3%) and 2240 (10%) were found to be 5% rare, of which 1595 (6.5%) and 1203 (5.4%) were found to be 1% rare (
Figure 9).
Three variants were of high quality, 5% rare, and matched a ClinVar pathogenic record. However, none of them matched a disorder related to the primary immunodeficiency observed in the subject (MSR1, rs72552387: Malignant tumor of prostate, SCN3B, rs121918282: Brugada syndrome 7, HAMP, rs104894696: Hemochromatosis, modifier of).
Two variants were found to be stringent high quality, coding or splicing, 1% rare homozygous, and disrupting a gene product with known immune phenotype association; both were frameshift substitutions, disrupting the genes MMP12 and ZFPM1 (chr11:102738794-102738795, MMP12:NM_002426:exon5:c.630_631AAA; chr16:88599700-88599705, ZFPM1:NM_153813:exon10:c.1334_1339CC). After close inspection, the MMP12 variant was found to be a likely reference sequence error or private variant (based on the HuRef sequence and other mammalian organisms sequence), whereas the ZPFM1 variants were in a region of dubious coding sequence accuracy.
Although it did not meet the stringent quality requirements, a potential compound heterozygous event was found for CHD4 and was prioritized for follow up because of a particularly interesting phenotype in the mouse model (abnormal T cell counts, hypoplastic thymus, abnormal spleen, liver inflammation); however, validation by Sanger sequencing failed, confirming the importance of focusing on variants with higher quality stringency.
Five variants were found to be stringent high quality, coding or splicing, 1% rare, heterozygous, and disrupting a gene product with known immune and dominant phenotype; however, none of them had clinical presentations compatible with the subject in study (TTN: hypertrophic cardiomyopathy, muscular distrophy; PLEC: epidermolysis bullosa, muscular distrophy; NUP214: acute leukemia; VWF: von Willebrand disease; ITGB3: bleeding disorder, posttransfusion purpura, Glanzmann thrombasthenia).
No rare (1%) CNV disrupted more than one gene, and none of the 6 genes with coding exons overlapped by 1% rare losses (OR5M10, FOLH1B, TRIM77, WFDC8) or 1% rare gains (ANKRD36, HYDIN) had any phenotypic or functional information implicating them in the patient's disorder.
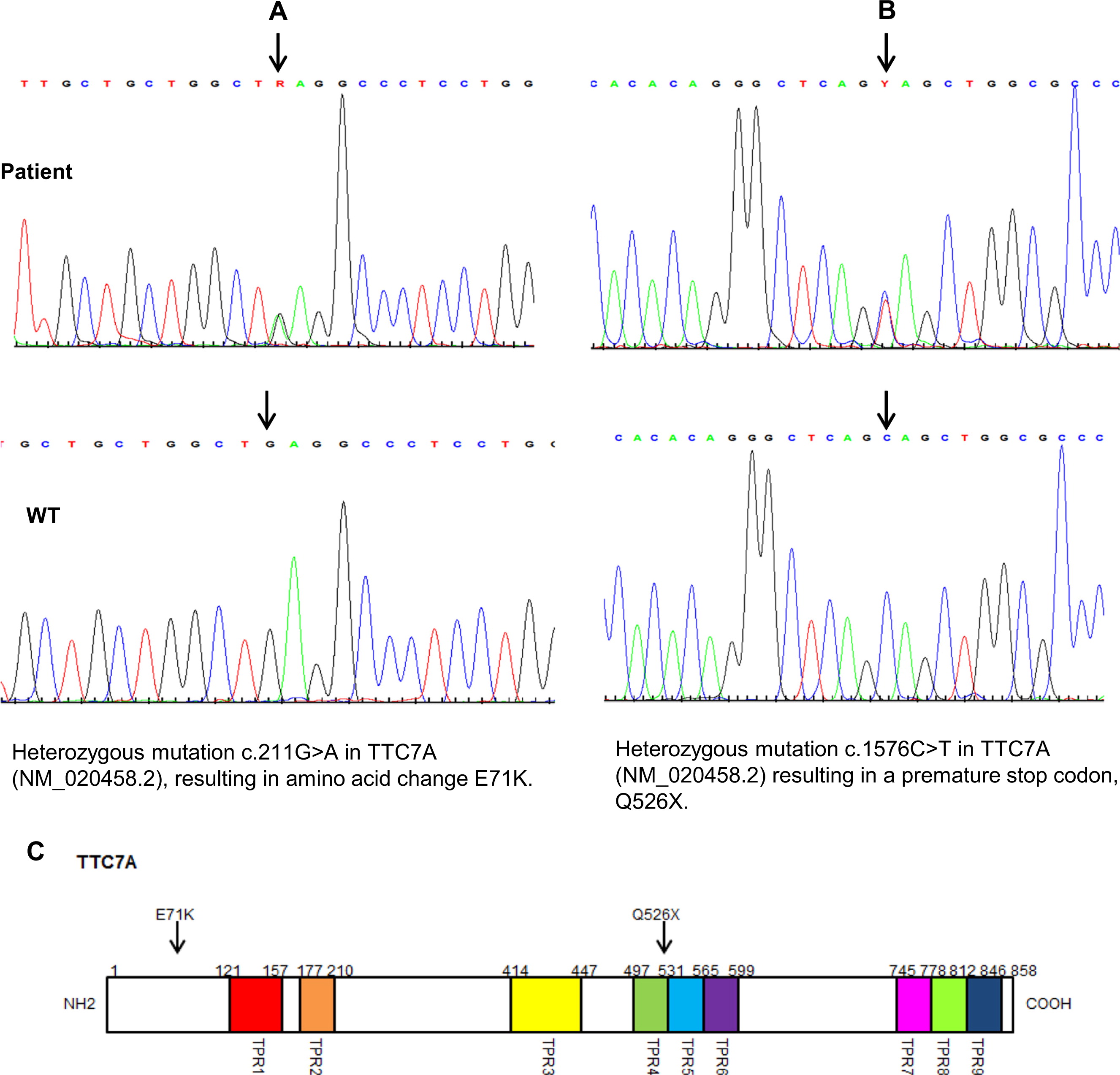
Only one gene, TTC7A, was found to have a potential compound heterozygous event composed of two or more variants in the stringent high quality group, coding or splicing, 1% rare homozygous, and disrupting a gene product with a known immune phenotype. Interestingly, the OMIM disorder associated with TTC7A is intestinal atresia in humans. The two variants, recognized as very low-frequency damaging missense and a novel stop-gain, were inspected carefully and were confirmed to have sufficient damaging potential (TTC7A:NM_020458:exon14:c.C1576T:p.Q526X).
Two mutations were confirmed by Sanger sequencing in the patient's and in her parents’
TTC7A gene. The mother carried a novel heterozygous nonsense mutation in exon 14 c.1576 C > T, a substitution that results in the premature termination of the protein at amino acid 526 (p.Q526X) (
Figure 10). The father was heterozygous for a G to A transition in exon 2 at position 211 (c 211 G > A), which predicts a glutamic acid to lysine substitution at position 71 (p. E71K). This mutation is likely to be deleterious (polyphen score 0.99) and located at the region predicted to be critical for protein–protein interactions (
Blatck and Lassle 1992;
Scheufler 2000).